NEUPRO® (rotigotine transdermal
system) is
indicated for the treatment
of Parkinson's disease (PD).
Helping patients
feel ready to
take
on the day
with NEUPRO.
Why NEUPRO?
NEUPRO improved activities
of daily living and motor
performance1

“When my PD motor symptoms bother me, I’m tired and not myself. It feels like I’m losing a part of who I am.”
– JANE, PD patient
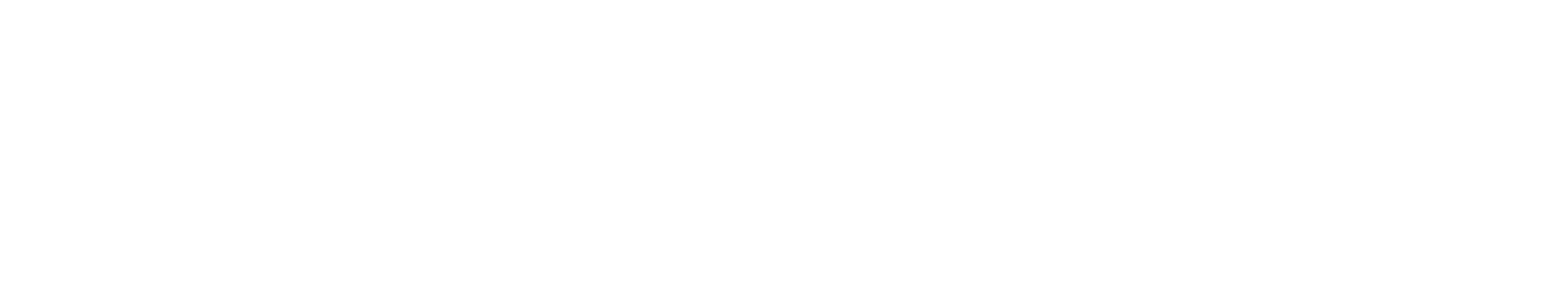
Activities of daily living and motor performance were measured by the UPDRS Parts II and III.1
- NEUPRO demonstrated a clinically relevant change in UPDRS Parts II and III scores of -5.3 over placebo.1,3
- The most common adverse reactions (at least 5% greater
than placebo) for NEUPRO in the treatment of PD are nausea,
vomiting, somnolence, application site
reactions, dizziness, anorexia, disturbances in initiating and maintaining sleep, hyperhidrosis, visual disturbance, peripheral edema, and dyskinesia.2 - Data reflect a 6-month, multicenter, multinational,
randomized, double-blind, placebo-controlled, prospective trial
in patients (n=277) with early-stage, idiopathic PD.
NEUPRO doses were titrated over 3 weeks to a maximum dose of 6 mg/24 h, followed by a 6-month maintenance phase. Primary outcomes were change from baseline
in UPDRS Parts II and III combined scores at end of maintenance.1

“When my PD motor symptoms bother me, I’m tired and not myself. It feels like I’m losing a part of who I am.”
– JANE, PD patient
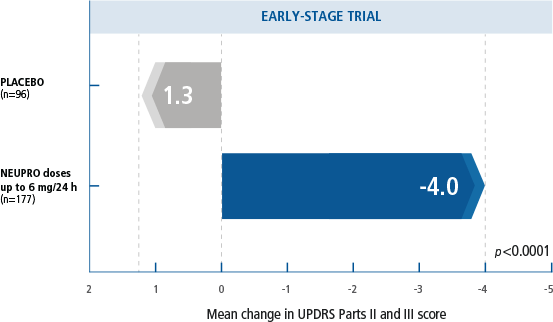
WHEN ADDED TO LEVODOPA
NEUPRO significantly
decreased “OFF” time for
PD patients2,4

“Because it’s a huge effort to get moving in the morning, I feel more tired throughout my day. How am I supposed to be able to get ready for work?”
– PAUL, PD patient
- Onset of treatment benefit started as early as the first week of treatment.2
- The most common adverse reactions (at least 5% greater
than placebo) for NEUPRO in the treatment of PD are nausea,
vomiting, somnolence, application site
reactions, dizziness, anorexia, disturbances in initiating and maintaining sleep, hyperhidrosis, visual disturbance, peripheral edema, and dyskinesia.2 - A multinational, three-arm, parallel-group study in
which 351 advanced-stage PD patients were titrated over 5 weeks
to treatment with either placebo or NEUPRO (8
mg/24 hours or 12 mg/24 hours*) and maintained treatment for 24 weeks followed by a down titration over the last week.2
*12 mg/24 h is not an approved dose.2
Patient Characteristics
- The mean daily intake of levodopa at baseline in the NEUPRO 8 mg/24 hours group was 760 mg/day vs 753 mg/day in the placebo group.4
- Patients were required to have ≥2.5 hours of daily “OFF”
time; the mean duration of daily “OFF” time at baseline was 6.8
hours in the NEUPRO 8 mg/24 hours group vs
6.4 hours in the placebo group.2,4 - All patients had a Hoehn and Yahr stage between II and IV in both the “ON” and “OFF” states.4
- 42% of patients included in the trial were <65 years old, 79% were <75, and 21% were ≥75.5

“Because it’s a huge effort to get moving
in the
morning, I feel more tired
throughout my day. How am I supposed
to be able to get ready for work?”
– PAUL, PD patient
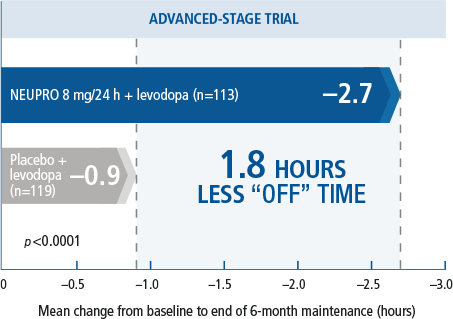
WHEN ADDED TO LEVODOPA
NEUPRO increased
functional “ON” time
without troublesome
dyskinesias4

“I’ve made lots of changes to remain as independent as I can for as long as possible, but things still get harder each day. The more I have to ask for help, the worse I feel about myself.”
- ALBERTO, PD patient
- NEUPRO may increase the dopaminergic side effects of levodopa and may cause or exacerbate pre-existing dyskinesia.2
- The most common adverse reactions (at least 5% greater than placebo) for NEUPRO in the treatment of PD are nausea, vomiting, somnolence, application site reactions, dizziness, anorexia, disturbances in initiating and maintaining sleep, hyperhidrosis, visual disturbance, peripheral edema, and dyskinesia.2
- A multinational, three-arm, parallel-group study in which 351 advanced-stage PD patients were titrated over 5 weeks to treatment with either placebo or NEUPRO (8 mg/24 hours or 12 mg/24 hours*) and maintained treatment for 24 weeks followed by a down titration over the last week.2
*12 mg/24 h is not an approved dose.2
Patient Characteristics
- The mean daily intake of levodopa at baseline in the NEUPRO 8 mg/24 hours group was 760 mg/day vs 753 mg/day in the placebo group.4
- Patients were required to have ≥2.5 hours of daily “OFF”
time; the mean duration of daily “OFF” time at baseline was 6.8
hours in the NEUPRO 8 mg/24 hours group vs
6.4 hours in the placebo group.2,4 - All patients had a Hoehn and Yahr stage between II and IV in both the “ON” and “OFF” states.4
- 42% of patients included in the trial were <65 years old, 79% were <75, and 21% were ≥75.5

“I’ve made lots of changes to remain
as independent
as I can for as long as
possible, but things still get harder
each
day. The more I have to ask for help, the
worse I feel about
myself.”
- ALBERTO, PD patient
NEUPRO offers 24-hour
continuous, stable drug
delivery2,6
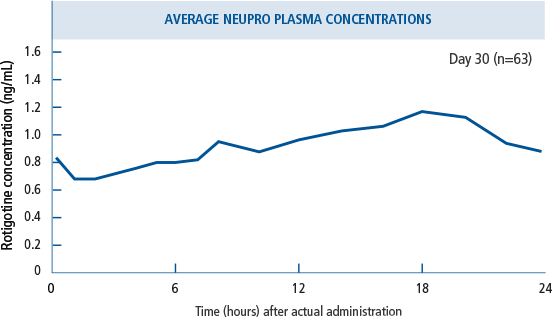
- NEUPRO is a transdermal system that provides continuous delivery of rotigotine for 24 hours following application to intact skin.2
- Relative bioavailability for the different application sites at steady-state was evaluated in subjects with PD. In a
single trial conducted in early-stage PD, differences
in bioavailability ranged from less than 1% (abdomen vs. hip) to 46% (shoulder vs. thigh) with shoulder application showing higher bioavailability.2
Setting patient
expectations
about dosing and titration
Because the NEUPRO Patch is available in 4 sizes and doses (2-8 mg) to treat PD, it’s important to help patients understand what to expect and to address their possible questions, such as “Will my dosage need to change?” You can let patients know that you may change their dose weekly based on their individual response and tolerability.2 They should also know it may take some time, possibly weeks, for them to feel the full effects of NEUPRO.2
Helping patients know what to
expect when they
start using the NEUPRO Patch is important for
their
treatment journey.
Early-stage PD
Initiate* the recommended starting dose
of
2 mg/24 hours.
Initiate* the recommended starting dose
of 4 mg/24 hours.
Advanced-stage PD
*Based upon individual patient clinical response and tolerability, dosage may be increased in weekly increments of 2 mg/24 hours until an effective dose is reached.2
Patches not shown at actual size.
For early-stage PD:
- The lowest effective dose is 4 mg/24 hours.2
- The maximum recommended dose is 6 mg/24 hours.2

For advanced-stage PD:
- The maximum recommended dose is 8 mg/24 hours.2
SETTING PATIENT EXPECTATIONS
About once-daily dosing
with the NEUPRO Patch2
There are some key things that patients will need to learn and remember to help them use their NEUPRO Patch properly.
Patients should be advised to read the Patient Information and Instructions for Use provided with their NEUPRO prescription before applying the patch.

The NEUPRO Patch should be applied at
approximately the same time
every day and worn for 24 hours. It should be removed and replaced
after the patient has worn it for 24 hours.2

The NEUPRO Patch should be applied to
clean, dry skin immediately
after opening the
pouch and pressed firmly in place for 30
seconds, with good contact around the edges.
The warmth of the
hand helps the adhesive
stick to the skin.2

The NEUPRO Patch should not be applied to the
same application
site more than once every 14 days.
It is
important to rotate the application site daily to reduce the
chance of skin irritations.2
SETTING PATIENT EXPECTATIONS
About adverse reactions
in early-stage PD
Adverse reactions in a placebo-controlled, fixed-dose trial in patients with early-stage PD
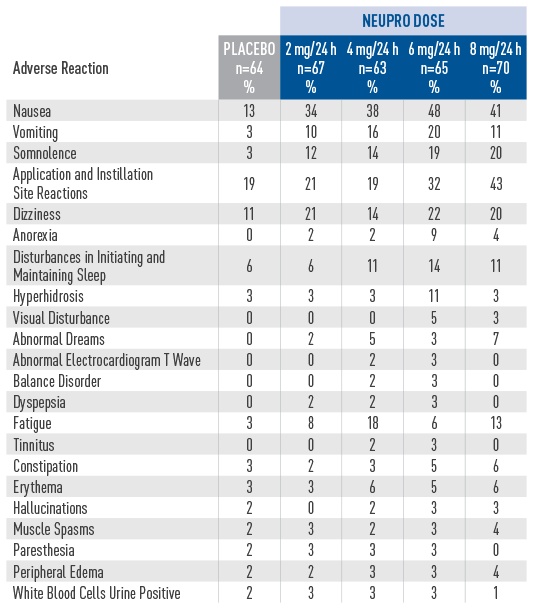
- The safety of NEUPRO was evaluated in a total of 649
early-stage PD patients who participated in three
double-blind, placebo-controlled studies with durations
of 3 to 9 months. Additional safety information was
collected in short-term studies and two open-label extension studies in patients with early-stage PD.2 - ln the double-blind, placebo-controlled, dose-response
study in patients with early-stage PD, the most common
adverse reactions (at least 5% greater than placebo) for
the maximum recommended dose of NEUPRO (6 mg/24
hours) were nausea, vomiting, somnolence, application
site reactions, dizziness, anorexia, disturbances in
initiating and maintaining sleep, hyperhidrosis, and
visual disturbance.2 - 12% of patients treated with the maximum
recommended NEUPRO dose (6 mg/24 hours)
discontinued treatment because of adverse reactions,
compared with 6% of patients who received placebo.2 - Adverse reactions that occurred in greater than 2% of
NEUPRO-treated patients and more frequent than in
placebo-treated patients are shown.2 - Incidences for the non-recommended 8 mg/24 hours
dose are also shown.2
SETTING PATIENT EXPECTATIONS
About adverse reactions
in
advanced-stage PD
Adverse reactions in a placebo-controlled, fixed-dose trial in patients with advanced-stage PD
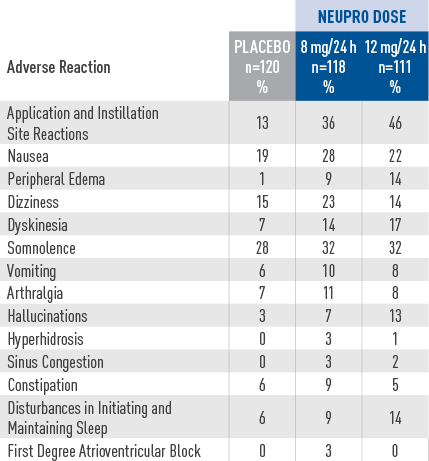
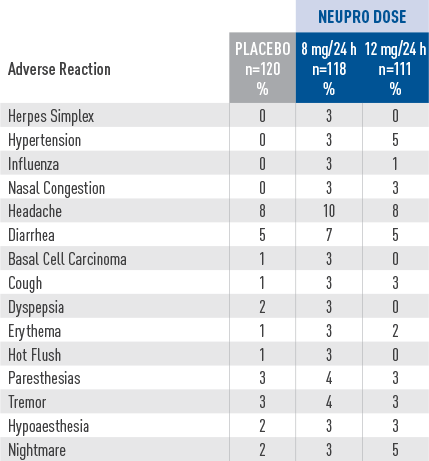
- The safety evaluation of NEUPRO was based on a total of
672 NEUPRO-treated patients with advanced-stage PD who
participated in three double-blind,
placebo-controlled studies (two fixed-dose trials and one flexible-dose trial) with durations of 3 to 7 months. Patients received concomitant levodopa in these
studies. Additional safety information was collected in earlier short-term studies and two open-label extension studies in patients with advanced-stage PD.2 - ln the dose-response, placebo-controlled trial for
advanced-stage PD, the most common adverse reactions (at least 5%
greater than placebo) for the maximum
recommended dose of NEUPRO (8 mg/24 hours) were application site reactions, nausea, peripheral edema, dizziness, and dyskinesia.2 - Approximately 15% of patients treated with the maximum
recommended NEUPRO dose (8 mg/24 hours) discontinued treatment
because of adverse reactions,
compared with 9% of patients who received placebo.2 - Adverse reactions that occurred in greater than 2% of NEUPRO-treated patients and more frequent than in placebo-treated patients are shown.2
- Incidences for the non-recommended 12 mg/24 hours dose are also shown.2
Resources are available to
help your patients learn
about
NEUPRO at
www.neupro.com.
As part of our commitment, NEUPRO offers a number of tools and resources to help patients with access to the medication and support throughout their treatment.
NEUPRO Patient Savings Program™
Eligible commercial patients may pay as little as $10 per 30-day supply of NEUPRO*
For ineligible patients, UCB may be able to help through the Patient Assistance Program,
which provides additional access to prescription coverage.
*Patients are not eligible if their prescriptions are paid in part or in full by any state or
federally funded programs. Other eligibility restrictions, terms and conditions apply.
Click here for details.

NEUPRO Patient Savings Card will expire at the end of the calendar year
Take a few minutes to watch
this video on how to apply
the NEUPRO Patch
For help with rotating the patch every day, patients may download the NEUPRO Patch Placement Tracker.
Other patient resources
Patients can go to www.neupro.com to download these resources and more and sign up for the Patch Partnership Program™